Building the Future of Cell Therapy: How AI and Robotics Are Revolutionizing Manufacturing
Building the Future of Cell Therapy: How AI and Robotics Are Revolutionizing Manufacturing
By modeling the condition space, AI successfully estimated the effective range of conditions (design space), enabling QbD (Quality by Design)-oriented studies from the early stages of process development.
In research and development for cell therapeutics, long and complex culture processes make quality and outcomes prone to variation, creating a major barrier to clinical application. To address this, Astellas Pharma collaborated with Robotic Biology Institute (RBI) and Epistra to pursue next-generation process development that fuses AI and robotics. What kinds of trials and iterations were undertaken to transcend the limits of conventional development? We spoke with Mr. Atsushi Inoue, Lead of Biological Technology, Drug Substance Research Labs, CMC Development at Astellas Pharma, who led this project, about the background and the results of the project.
Variability in Cell Culture Was the Wall in Process Development
— To begin, could you tell us about the background and challenges when you launched the project?
The fundamental and serious issue that motivated this project was securing reproducibility in cell culture. Because cells are living materials, even minor differences in handling can have a large impact on results. Over culture periods that span several weeks to months, those subtle differences accumulate and significantly alter the final outcome.
Moreover, the drivers of variation are often tiny, undocumented differences in technique or judgment, which makes it difficult to pinpoint root causes when problems arise. As a result, there were inevitable limits in both development speed and consistency with conventional methods.
To tackle this, we first introduced robots to standardize techniques and built a highly reproducible culture environment in which anyone can obtain the same result, regardless of who performs the work, when, or how. This robotic platform was originally developed by Astellas in collaboration with RBI, and we leveraged that foundation for this project.
However, to fully realize the platform’s potential, we believed it was essential to pair its high-precision data with AI capable of utilizing it. We therefore brought in Epistra—who have deep expertise and a strong track record in AI-driven condition optimization—and launched a full-scale, three-party collaboration.
Breaking Through the Variability Barrier with Lab Automation
— Pursuing a reproducible process that doesn’t rely on individual experts was your starting point. What practical changes did robot adoption bring?
Our culture periods exceed 90 days, and it’s simply not realistic to manually maintain perfectly consistent operations throughout that time. Because slight differences in timing or conditions directly affect results, a robot’s ability to perform precise, consistent operations continuously is indispensable. In manual culture experiments, CVs (coefficient of variations) exceeding 20% are not uncommon. In contrast, in the preliminary studies we performed with robots for this project, we achieved a CV of 5.9%, demonstrating very high reproducibility. Achieving this “deviation-free culture” was a crucial foundation for the entire process development effort.
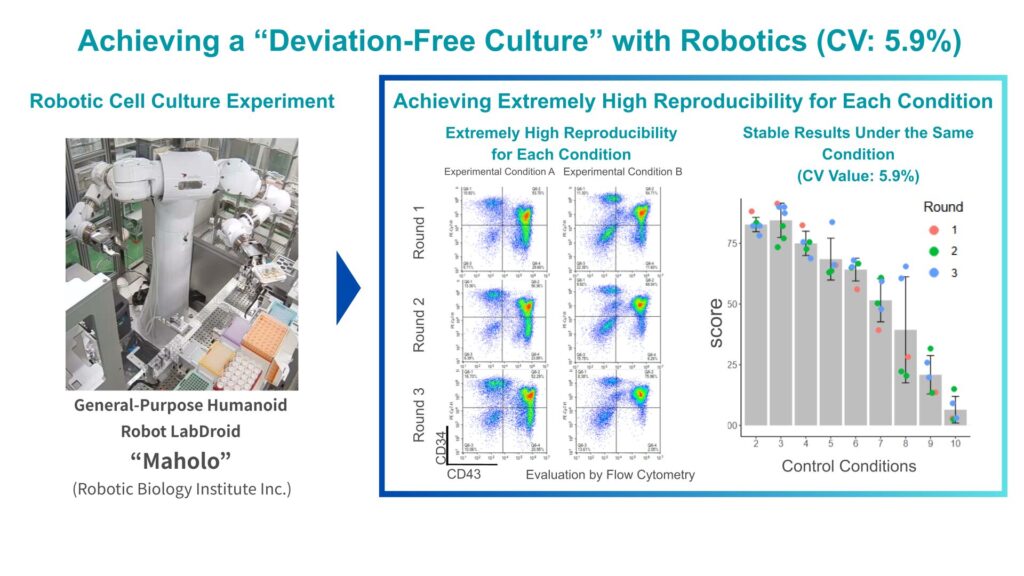
Better Data, Better AI — The Synergy of Robots and AI
— Once you had that stable foundation, you moved to AI-driven optimization. What results did the AI deliver?
This was the turning point that we believe will reshape process development. With conventional approaches, one must rely on prior experience and limited publications, iterating through trial and error to locate optimal conditions. Yet long culture processes present a vast number of parameters, and exploring that high-dimensional space with traditional trial-and-error methods felt inherently limited.
When we used Epistra’s AI to explore conditions, the results far exceeded expectations. Within three months, we identified multiple conditions that produced 50 to 100 times the yield reported in the scientific literature.
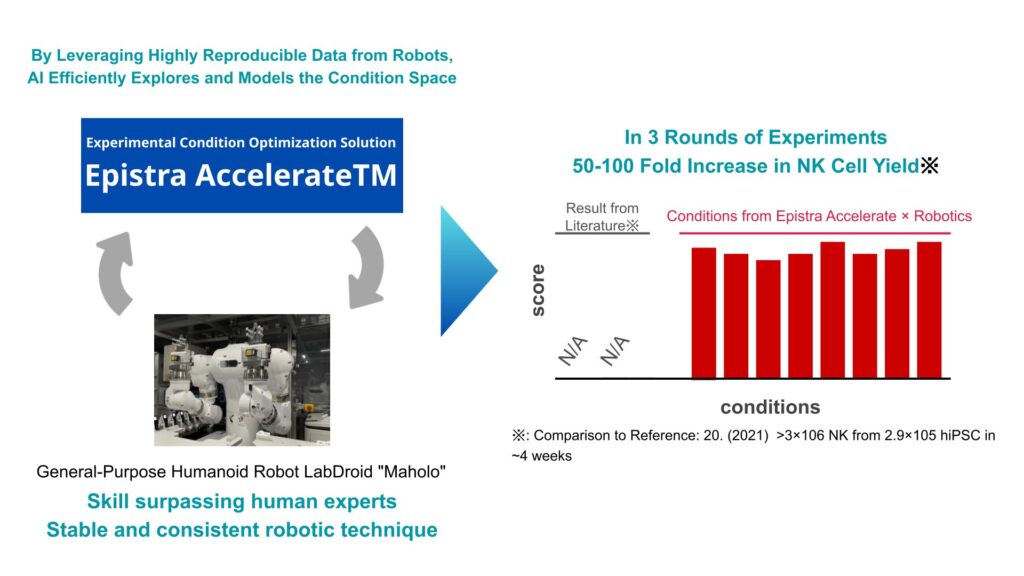
This suggests we can dramatically accelerate what used to be multi-year process-development efforts by proceeding efficiently with a limited number of experiments. The new AI-plus-robotics approach clearly translated into tangible results, and we believe it sets a new direction for future work.
(Ozawa): AI alone has limits, but in combination with robots we can consistently obtain high-precision, highly reproducible data—substantially improving AI model accuracy. This synergy is what led to these results. We strongly felt that pairing low-variance, high-quality data with AI capable of efficiently exploring high-dimensional spaces is extremely powerful for process development in cell therapeutics.

Right: Atsushi Inoue, Astellas Pharma
Left: Yosuke Ozawa, CEO, Epistra
A New Approach: QbD-Aligned Studies from the Start with AI + Robotics
— Beyond your initial expectations, were there any surprising developments or memorable findings?
One notable point is that Epistra’s AI enabled us to estimate the design space—the effective range of conditions—right from the very early stage of process development. Conventional statistical methods are used as well, but they’re constrained in the number of factors and experiments they can handle, making it difficult to survey a wide area at once. And in early design phases, one must narrow the scope based on hypotheses, which limits exploratory flexibility.
In this project, the AI learned structures—including inter-factor relationships—from limited experimental data and automatically estimated the design space. Of course, further validation is needed to confirm those estimates, but incorporating a Quality by Design (QbD) approach from the earliest stages of development was a major achievement.
QbD, promoted by regulators including the FDA, is increasingly important in designing GMP-compliant manufacturing processes. We believe this new AI-and-robotics approach can be an effective way to realize QbD earlier in development. Building on these results, we’d like to continue exploring how AI can support decision-making from the earliest design stages.
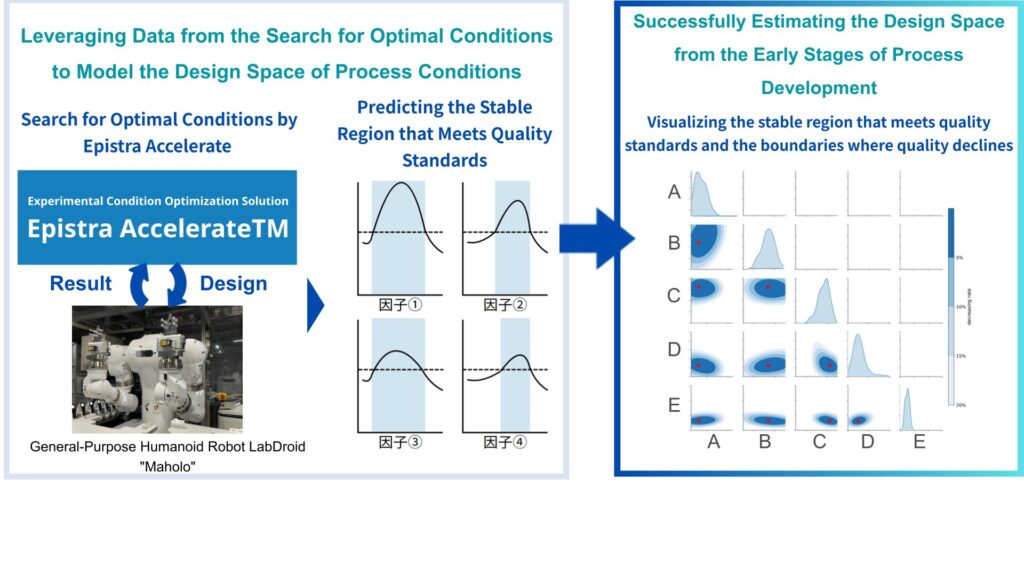
(Ozawa): We’re honored to hear that—this is precisely what we set out to achieve. Epistra’s AI is based on Bayesian optimization. Behind the optimization lies a predictive model known as a Gaussian Process (GP), which learns the shape of the underlying function from observations, estimating the expected outcome and uncertainty at each set of conditions. This means we can do more than find a single optimum—we can probabilistically estimate the regions (i.e., the design space) where good results are likely.
Scaling the AI × Robotics Framework to Bring Cell Therapeutics to Patients

— What kind of response have you seen to these results?
We’ve received a very strong response inside and outside the company—many people have said, “We want to use this in our project,” or “We’d like to learn how you’re doing it.” In particular, the combination of low-variance, highly reproducible data and rapid yield improvements far beyond published reports drew widespread attention. The 5.9% CV and stability across lots overturned our prior assumption that “cells can’t be fully controlled.”
— How do you plan to deploy this framework going forward?
As our press release notes, we plan to roll out this process-development framework—combining LabDroid “Maholo” and AI—as a manufacturing platform for cell-based medical products. We aim to support academia and startups by providing our platform for their cell-therapy seeds and building a system that supports everything from process development to GMP-compliant manufacturing.
One barrier to the practical use of cell therapeutics is the gap where promising seeds exist, but a reproducible process cannot be established. Our initiative offers one answer: a new process-development approach that combines robotics for reproducibility with AI for condition optimization.
To make regenerative medicine a reality in society, we must go beyond simple automation and establish mechanisms that stably maintain cell quality and ensure sustainable supply. That requires confronting the core challenges from the earliest stages and using AI to narrow conditions efficiently. We believe such efforts will only accelerate as AI technologies continue to evolve.
By advancing initiatives like this one, we can move step by step toward the social implementation of regenerative medicine, while also advancing manufacturing to deliver therapies more reliably to patients. To make that change real, we must not keep these technologies confined within a single company, but return them to society in forms that many researchers and developers can use. That, we believe, is our role in making next-generation cell therapeutics a reality—and ultimately delivering them to patients. We are fully committed to pursuing that future.
About the Interviewee
Atsushi Inoue
Lead, Biological Technology
Drug Substance Research Labs, CMC Development
Astellas Pharma Inc.
